Private-label topical (PLT) analgesics are independently manufactured lotions and creams that are increasingly being prescribed to patients for the temporary relief of minor pain associated with injury. The expensive products are often marketed by manufacturers as having unique formulations and special ingredient blends. In reality, most of these products often contain ingredients that can be found in inexpensive, widely accessible over-the-counter (OTC) topical products.
PLTs are reviewed by the FDA for their safety and efficacy
While the individual ingredients in PLTs may be approved by the FDA, the combined formulations have not necessarily undergone controlled studies and have not been approved by the FDA.
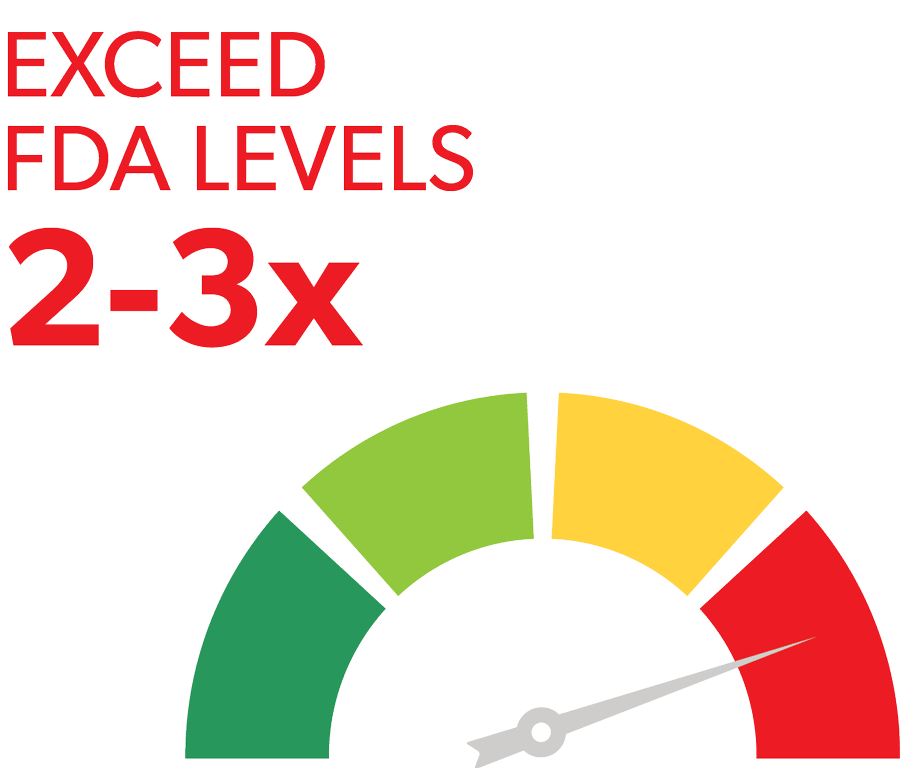
PLTS OFTEN EXCEED FDA THRESHOLDS FOR INGREDIENT LEVELS BY 2-3X, INCREASING RISK FOR SKIN BURNS1,2
PLTs are more effective than OTC lotions and creams
PLTs’ ingredient makeup often overlaps heavily with OTC topical products.
PLT: MEDROX® OINTMENT
$750/240 GM
OTC: ZIKS CREAM $26/240 GM

PLTs don’t have a significant impact on overall pharmacy costs
Although PLTs don’t represent a large portion of workers’ comp prescriptions, their high average wholesale price (AWP)/ unit cost makes a significant impact when they appear in claims.
PLT AWPS OFTEN EXCEED $500.1 COMPARABLE OTC PRODUCTS (E.G., BENGAY®, ICYHOT®) TYPICALLY RETAIL FOR <$10
